While telisotuzumab adizutecan demonstrated early antitumour activity, only modest benefits were seen with VCN-01 added to standard care
Antibody–drug conjugates (ADC) continue to build momentum and their potential application in metastatic pancreatic ductal adenocarcinoma (PDAC) has generated increasing attention due to limited second-line options and a poor prognosis with approved treatments (Lancet. 2016;387:545–557). Encouraging results were observed with telisotuzumab adizutecan, a c-Met protein-targeting ADC, which resulted in a response in almost one-quarter of pre-treated patients with advanced or metastatic PDAC in a phase I basket study presented at the ESMO Congress 2025 (Berlin, 17–21 October) (Abstract 2214MO).
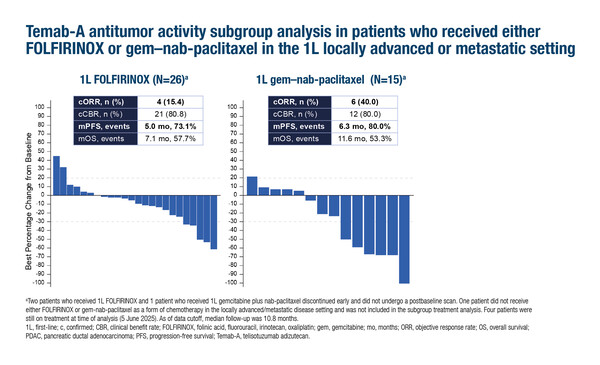
In the study, the confirmed objective response rate (ORR) was 23.8%, median duration of response was 6.9 months and median progression-free survival (PFS) was 5.4 months in 42 patients who received telisotuzumab adizutecan in the second-line setting. The confirmed ORR was 40% in the 15 patients who received first-line gemcitabine–nab-paclitaxel (median PFS of 6.3 months) and 15.4% in the 26 patients who received prior FOLFIRINOX (median PFS of 5.0 months). Treatment-related adverse events (TRAEs) led to discontinuation in 14% of patients, dose interruptions in 38% and dose reductions in 48% of patients. Immunohistochemical analysis showed that all tested tumour tissues had c-Met protein staining with 2+ and/or 3+ intensity.
“The seemingly worse efficacy in patients pre-treated with FOLFIRINOX may be expected given the topoisomerase I inhibitor payload,” says Prof. Ignacio Garrido-Laguna from the Huntsman Cancer Institute and University of Utah Health Care, Salt Lake City, UT, USA. “A higher percentage of patients had received prior FOLFIRINOX than is typical. Also a lower percentage of patients had liver metastases compared to other trials (N Engl J Med. 2013;369:1691–1703; N Engl J Med. 2011;364:1817–1825), which perhaps suggests some patient selection that may impact time-based endpoints.” He highlights that the relationship between MET expression and response will be interesting to evaluate further and adds: “There is currently considerable interest in KRAS inhibitors in this second-line setting. One could envisage a place for this ADC beyond progression to KRAS inhibitor as one of the proposed mechanisms of resistance to KRAS inhibitors is MET overexpression.”
According to current rates, patients with advanced PDAC live for under a year with approved first-line treatments (N Engl J Med. 2013;369:1691–1703; N Engl J Med. 2011;364:1817–1825) and there is a need to enhance treatment strategies with, for instance, the use of oncolytic adenoviruses to improve chemotherapy penetrance and have direct antitumour effects. As reported in Berlin, modest benefits were observed with VCN-01 (zabilugene almadenorepvec), an oncolytic adenovirus designed to replicate in cells with dysfunctional RB1 pathways and express hyaluronidase (PH20) to disrupt pancreatic cancer stroma (J Immunother Cancer. 2022;10:e003255). In the VIRAGE phase IIb trial, an ORR of 39.6% was observed when up to 2 doses of VCN-01 were added to gemcitabine–nab-paclitaxel versus 31.3% with gemcitabine–nab-paclitaxel alone in the full analysis set (FAS) of 96 patients with newly diagnosed metastatic PDAC (Abstract 2216MO). The primary endpoint of median overall survival (OS) in the FAS was 10.8 months in the VCN-01 plus chemotherapy arm versus 8.6 months in the chemotherapy alone arm (hazard ratio 0.57; 95% confidence interval 0.34–0.96; Cox p=0.034; log-rank p=0.055). The most common grade ≥3 TRAEs after the first VCN-01 dose were transaminase increases (15.1%) and influenza-like illness (13.2%).
In trials using centralised review – MPACT and the French consortium study – ORRs of 23% and 32% were observed with current first-line chemotherapy (N Engl J Med. 2013;369:1691–1703; N Engl J Med. 2011;364:1817–1825). “There appeared to be some benefit of adding the adenovirus to standard care but similar ORRs have been reported in other studies in PDAC,” explains Garrido-Laguna. “Perhaps the PFS analysis is limited due to imbalances between treatment arms with a higher percentage of patients with liver metastases in the control arm (83% versus 65%). Although the hypothesis of targeting hyaluronidase is interesting, there is a precedent of a negative randomised phase III trial with PEGPH20, a recombinant human hyaluronidase (J Clin Oncol. 2020;38:3185–3194).”
Programme details:
Harding JJ, et al. Phase 1 basket study of telisotuzumab adizutecan (ABBV-400; TEMAB-A), a c-MET protein–targeting antibody-drug conjugate: results from patients (pts) with pancreatic ductal adenocarcinoma (PDAC). ESMO Congress 2025 - Abstract 2214MO
Garcia-Carbonero R, et al. VIRAGE trial: Randomized Phase IIB, open-label, study of nab-paclitaxel and gemcitabine with/without intravenous VCN-01 in patients with metastatic pancreatic cancer (mPDAC). ESMO Congress 2025 - Abstract 2216MO