Findings from four studies provide further evidence on the potential of AI-driven pathology, imaging and multiomic data to guide clinical decision-making
Artificial intelligence (AI) research in oncology continues at a sustained pace, as evidenced by the volume of abstracts presented at the ESMO Congress 2025 (Berlin, 17–21 October) exploring the potential of AI technology for determining prognosis and predicting treatment response in cancer. “We are witnessing a paradigm shift toward personalised medicine, where imaging and blood-based biomarkers help guide care more precisely and safely,” remarks Dr Fátima Al-Shahrour from the Centro Nacional de Investigaciones Oncológicas, Madrid, Spain. “While some assays, such as circulating tumour (ct)DNA, remain largely in the research domain, other technologies, including AI analysis of computed tomography (CT) scans and pathology slides, are already being implemented in clinical practice.”
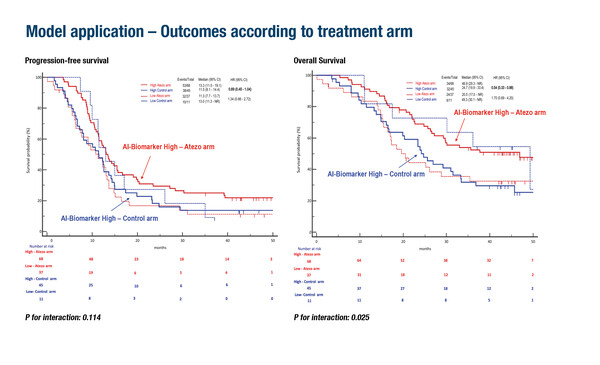
This is exemplified in an AI-driven biomarker predictive model developed to predict clinical benefit from adding atezolizumab to FOLFOXIRI–bevacizumab in 161 patients with proficient mismatch repair metastatic colorectal cancer in an analysis of the AtezoTRIBE study (Abstract 725O, key results in the box below). Addition of atezolizumab to FOLFOXIRI plus bevacizumab resulted in longer respective median progression-free survival (mPFS; 13.3 months versus 11.5 months) and overall survival (mOS; 46.9 months versus 24.7 months) than FOLFOXIRI plus bevacizumab in patients classified as biomarker-high based on AI analyses of whole-slide images. No benefit of atezolizumab was observed in biomarker-low patients. These findings were validated in an analysis of the AVETRIC study (n=48), where biomarker-high patients treated with mFOLFOXIRI plus cetuximab plus avelumab had longer respective mPFS (16.3 months versus 10.8 months) and mOS (40.0 months versus 29.4 months) than biomarker-low patients.
“This approach could be seamlessly integrated into pathology workflows, helping clinicians avoid unnecessary toxicities and adapt treatment for patients unlikely to benefit,” says Al-Shahrour. “AI allows us to go beyond what the human eye can detect in histology slides, quantifying the tumour microenvironment to identify patients most likely to respond to immune checkpoint inhibitors.”
AI-based imaging analysis and multiomics successfully predicted outcomes for patients with relapsed mesothelioma treated with niraparib in the NERO trial (LBA106, key results in the box below). CT scans were analysed with the AI model ARTIMES for 85 patients and archival tissue was sequenced to quantify intratumour heterogeneity (ITH). Pre-treatment tumour volume was shown to be prognostic for OS (p=0.01) and significantly longer PFS was observed with niraparib versus active symptom control (hazard ratio [HR] 0.19; 95% confidence interval [CI] 0.06–0.58; p=0.003) in patients with high ITH but not in those with low ITH (HR 1.40; 95% CI 0.55–3.60; p=0.482). As Al-Shahrour explains, “Mesothelioma is a notoriously difficult disease to measure and standard RECIST assessments are often inaccurate and subjective. Here, the AI model ARTIMES provided an objective, reproducible way to quantify tumour volume from routine CT scans. When combined with genomic data (ITH measure), it helped identify which patients were most likely to respond to PARP inhibitors.” She adds: “This dual AI-genomic approach has multiple applications – firstly to predict prognosis and secondly to select patients likely to benefit from specific treatments.”
AI-driven imaging biomarkers were shown to predict treatment response and outcomes in non-small cell lung cancer (NSCLC) across two studies. In exploratory analyses of the AEGEAN trial (n=111), changes in radiomic features from screening to surgery predicted complete pathological response (area under the curve [AUC] 0.82) (LBA70, key results in the box below). Adding ctDNA status at cycle 3, day 1 improved the prediction slightly (AUC 0.84), and radiomic findings, with or without ctDNA, were also associated with event-free survival. In a post hoc analysis of the CROWN trial, ALK-positive patients with baseline brain metastases (n=70) were stratified into low- versus high-risk groups based on AI-derived early responses in brain lesions, with significantly longer mPFS in the low-risk group (33.3 months versus 7.8 months in the high-risk group) (Abstract 2012P, key results in the box below). AI-derived lung tumour features also significantly predicted PFS in patients without baseline brain metastases (n=179). By contrast, RECIST results at first follow-up did not significantly predict PFS in either subgroup. “These studies are very promising,” says Al-Shahrour. “By combining non-invasive CT imaging with AI-driven metrics, and in some cases ctDNA, we can identify early which patients are likely to respond, refine treatment decisions and avoid delays or unnecessary invasive procedures.”
Al-Shahrour concludes: “Across these studies, AI consistently enhanced our ability to measure, predict and personalise treatment response. Whether through digital pathology, imaging or multiomics, we are moving toward a data-driven model of oncology where algorithms complement clinical judgment.”
At a glance:
Carullo M, et al. Leveraging artificial intelligence to predict immune checkpoint inhibitor (ICI) efficacy in proficient MMR mCRC: translational analyses of AtezoTRIBE and AVETRIC trials. ESMO Congress 2025 - Abstract 725O
- AtezoTRIBE (N=161)
- Biomarker-high classification: 70% pts
- In atezo arm, biomarker-high pts had superior PFS (p=0.036) and OS (p=0.024) vs biomarker-low pts
- For biomarker-high (but not biomarker-low) pts, interactions observed between treatment and biomarker for PFS (HR 0.69) and OS (HR 0.54)
- AVETRIC (validation set; N=48)
- Biomarker-high classification: 75% pts
- Biomarker-high pts had superior PFS (p=0.043) and OS (p=0.053) vs biomarker-low pts
Fennell DA, et al. 3D-response and multiomic correlates of niraparib in relapsed mesothelioma: NERO, a randomised clinical trial in patients with relapsed mesothelioma. ESMO Congress 2025 - LBA106
- Pre-treatment changes in mesothelioma volume were prognostic: OS, Spearman's R=0.24; p=0.01
- Intratumour heterogeneity predicted response to niraparib: weighted Shannon entropy (WSE)high PFS (niraparib + active symptom control [ASC] vs ASC): HR 0.19 (p=0.003)
Heymach J, et al. Association of radiomic features ± on-treatment ctDNA detection with treatment outcomes in patients with resectable NSCLC: exploratory analyses from AEGEAN. ESMO Congress 2025 - LBA70
- Radiomic features:
- Predicted pCR: AUC 0.82 (radiomics)
- Predicted that 33% and 18% of pts in the durvalumab and placebo arms, respectively, were more likely to have pCR
- Radiomic features + ctDNA status:
- Predicted pCR: AUC 0.84
- Predicted that 30% and 18% of pts in the durvalumab and placebo arms, respectively, were more likely to have pCR
Lu S-L, et al. A post-hoc analysis of the CROWN Study in ALK+ NSCLC using AI to predict progression-free survival based on early response. ESMO Congress 2025 - Abstract 2012P
- N=249
- Median follow-up: 3 y
- AI-derived early brain metastases (BM) response predicted systemic PFS:
- Low- vs high-risk groups (33.3 mo vs 7.8 mo; HR 0.34; p=0.0006)
- In pts without baseline BMs, lung tumour features predicted PFS:
- Low- vs high-risk groups (NR mo vs 11.1 mo; HR 0.44; p=0.0001)