High rates of pathological complete response were reported with neoadjuvant datopotamab deruxtecan plus durvalumab in patients with specific subtypes of breast cancer and at high risk of recurrence
Neoadjuvant datopotamab deruxtecan plus durvalumab was associated with rates of pathological complete response (pCR) – the primary endpoint – of 79% in patients with immune-positive signatures and 62% in those with hormone receptor (HR)-negative/HER2-negative breast cancer enrolled in the I-SPY 2.2 trial (LBA15), as reported at the ESMO Congress 2024 (Barcelona, 13–17 September). More than 50% of the reported pCR in these subgroups of patients were achieved after datopotamab deruxtecan plus durvalumab alone, thus avoiding the need for taxane- and anthracycline-based regimens.
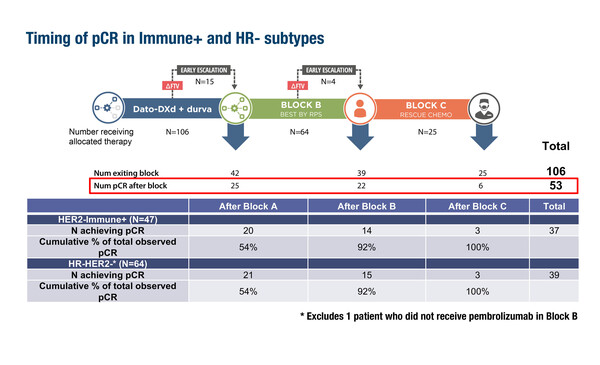
I-SPY 2.2 has an adaptive trial design that sequentially tests up to three neoadjuvant treatments (Blocks A, B and C) with a primary endpoint of pCR. Specific regimens within Blocks B and C are selected according to tumour response predictive subtypes (RPS). RPS incorporates tumour signatures based on factors such as immune response, DNA repair deficiency (DRD), and HR and HER2 status. Patients were initially randomised to datopotamab deruxtecan plus durvalumab (Block A). Those patients who were predicted to respond according to MRI and biopsy investigation could undergo surgery early, otherwise, they proceeded to the next treatment (Block B; a taxane-based regimen selected according to RPS). Patients who were predicted to respond to Block B treatment could undergo surgery whereas those not predicted to respond progressed to Block C, where they received rescue chemotherapy (doxorubicin plus cyclophosphamide with or without pembrolizumab). Estimated pCR rates were compared to a subtype-specific dynamic control (DC) from historical I-SPY data.
Overall, 106 patients with HER2-negative disease were enrolled in the study and an overall pCR of 50% was achieved. Among patients with immune-positive signatures or HR-negative disease, 37 (70%) and 39 (62%) patients, respectively, achieved pCR. For both of these subgroups, the cumulative percentage of total observed pCR was 54%, 92% and 100% after treatment in Blocks A, B and C, respectively. For patients with tumour subtype negative for HR, immune signature and DRD, the modelled pCR rates after all treatments (Blocks A, B and C) performed better than the DC.
Programme details
Trivedi MS, et al. Rates of pathologic complete response after datopotamab deruxtecan plus durvalumab in the neoadjuvant setting: Results from the I-SPY 2.2 trial. ESMO Congress 2024, LBA15
Mini Oral Session – Breast cancer, early stage, 14.09.2024, h. 10:15 – 11:55, Barcelona Auditorium – Hall 2